Guanine-nucleotide exchange factors (GEFs)
GEFs (guanine-nucleotide exchange factors) activate small G proteins (GTPases) such as Rac, which regulates cell shape, motility, oxygen radical formation and gene expression (Fig 1). Rac can be activated by several types of Rac-GEFs, including the P-Rex family.
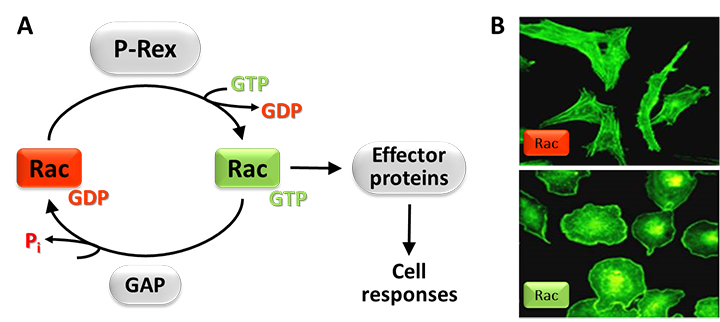
Figure 1. Rac-GEFs.
A) Small G proteins like Rac are active when GTP-bound and inactive when GDP-bound. Rac-GEFs such as P-Rex activate Rac by exchanging GDP for GTP, enabling Rac to bind various effector proteins that stimulate cell responses. Rac signals are terminated by GAPs. (B) Effect of active Rac on cell shape. The photos show endothelial PAE cells without (top) or with (bottom) active Rac, stained with fluorescent phalloidin to label the cytoskeletal protein F-actin.
P-Rex family
Our lab discovered the P-Rex (PIP3-dependent-Rac exchanger) family of Rac-GEFs, which consists of P-Rex1, P-Rex2 and the splice variant P-Rex2b. P-Rex family Rac-GEFs are important for a wide array of cell functions. Their physiological roles include the control of leukocyte responses that safeguard our immunity against bacterial and fungal infections, the regulation of neuronal morphology and synaptic plasticity that are required for motor coordination and for social interactions, the migration of melanocytes that regulates skin pigmentation during development, the insulin signalling and glucose transport processes that regulate blood glucose levels, and the heat-generating capacity of brown fat tissue. Deregulated levels or activity of P-Rex family Rac-GEFs are often seen in cancer, where they contribute to tumour growth and metastasis, and they have also recently been described in insulin resistance and in autism.
P-Rex1 in inflammation and infection
P-Rex1 regulates the pro-inflammatory responses of leukocytes that confer our immunity against bacterial and fungal infections. Recently, we showed that the P-Rex1 in blood platelets, together with the Rac-GEFs Vav1 and Vav3, controls the interactions of platelets with leukocytes and with the blood vessel wall which enable leukocytes to migrate out of the blood stream into inflamed and infected tissues. The GEFs are required for the upregulation of the selectin ligand PSGL1 on the platelet surface, for the formation of platelet/neutrophil complexes in the circulation, and for the adhesion of neutrophils to the inflamed vessel wall under inflammatory conditions (Fig 2).
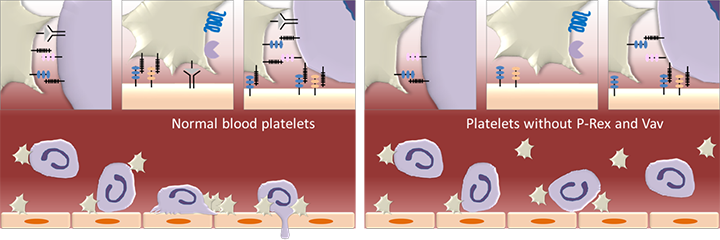
Figure 2. P-Rex1 in leukocyte recruitment.
P-Rex1 in blood platelets, together with the Rac-GEFs Vav1 and Vav3, is required for the interactions between platelets and neutrophils in the circulation and with the endothelial cells that line the wall of blood vessels. These interactions are required for the recruitment of neutrophils and other leukocytes from the blood stream into inflamed and infected tissues. Platelets are depicted in beige, neutrophils in purple and vascular endothelial cells in orange. (Figure is adapted from Pitchford S et al. 2017, Curr Opin Hematol 24, 23-31)
P-Pex1 and P-Rex2 in the nervous system
P-Rex1 is present throughout the nervous system and P-Rex2 in the Purkinje neurons of the cerebellum. We showed that Purkinje neurons which lack P-Rex2 have perturbed dendrite morphology and synaptic plasticity (Fig 3). The function of Purkinje neurons is to coordinate our motor skills. Accordingly, P-Rex2-deficient mice have impaired motor control which worsens during aging, and this impairment is exaggerated when P-Rex1 is also deleted. Recent work by others showed that cellular levels of P-Rex1 are altered in autistic patients. Studies in mouse models of autism showed that P-Rex1 is required for the synaptic plasticity of hippocampal neurons, which is an important cellular basis for social interactions. Importantly, restauration of normal P-Rex1 levels in the hippocampus of these mice restored their capacity for social interactions.
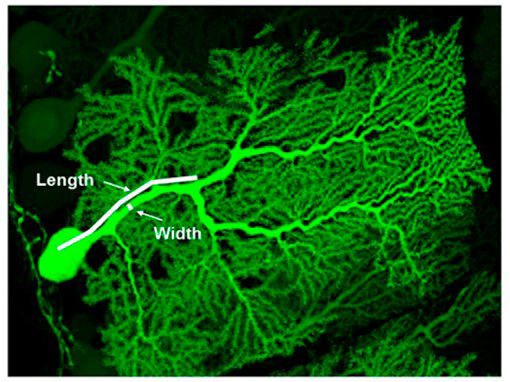
Figure 3. P-Rex2 controls the shape and function of Purkinje neurons.
High-resolution confocal image of a cerebellar Purkinje neuron expressing green fluorescent protein but deficient in P-Rex2. The size of the principal dendrite and the capacity for long-term potentiation of these cells are reduced. Purkinje neurons control movement, and motor coordination is impaired in P-Rex2 deficient mice (from Donald S et al, 2008, PNAS 105, 4483-8).
P-Rex regulation
P-Rex family GEFs are coincidence detectors for the activation of G protein coupled receptors (GPCRs) and of receptors that signal through phosphoinositide 3-kinase (PI3K) (Fig 3). These GEFs are activated by binding the lipid second messenger PIP3 that is produced by PI3K and by binding the Gβγ subunits of heterotrimeric G proteins which are released upon GPCR stimulation. In addition, their catalytic activity is modulated by various phosphorylation events, through protein kinases and phosphatases, as well as by binding the GPCR adaptor protein Norbin. Binding to PIP3, Gβγ and Norbin also induces the translocation of the usually cytosolic P-Rex to the plasma membrane of the cell, where the GEFs need to be localised in order to activate Rac. In addition, P-Rex2, but not P-Rex1, has a GEF-activity independent adaptor function which inhibits the tumour suppressor PTEN, and its catalytic activity, in turn, is also regulated by PTEN.
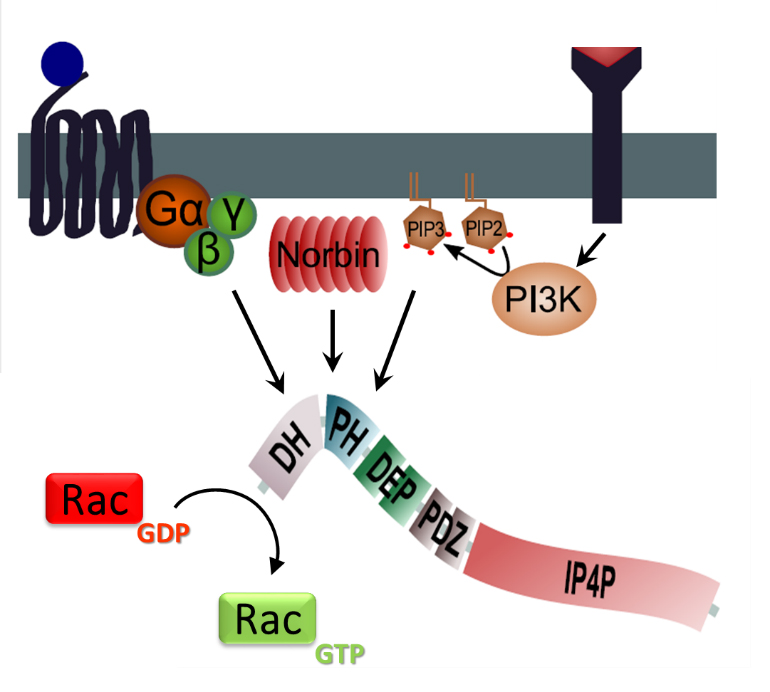
Figure 4. P-Rex regulation.
PIP3 and Norbin binding to the PH domain of P-Rex, and Gβγ binding to the catalytic DH domain, induce P-Rex localisation at the plasma membrane and enable P-Rex to catalyse the exchange of GDP for GTP in Rac. P-Rex phosphorylation by the kinase PKA inhibits its GEF activity, and other phosphorylation events modulate the activity. P-Rex2 activity is also regulated by binding to the tumour suppressor PTEN. (Figure adapted from Hornigold K et al, P-Rex1, Encyclopedia of Signaling Molecules, Springer. In Press)
New ways of monitoring Rac-GEF activity
We recently generated a reporter mouse strain that allows us to track Rac activity in living cells and tissues without altering cell responses. We have been using this reporter strain to monitor Rac activity in the intestine, liver, pancreas, skin and mammary tissue, both in response to stimulation or inhibition of Rac activity and upon genetic manipulation of various upstream regulators of Rac pathways, which revealed unexpected insights into Rac signalling during disease development. We have also used this reporter strain to monitor Rac activity in chemotaxing neutrophils and found enrichment of active Rac at the leading-edge, as well as unexpected shifts and oscillations (Fig 5). Currently, we are using the reporter strain to identify Rac-GEFs that activate specific subcellular pools of Rac activity which are required for neutrophil adhesion, migration and phagocytosis.
Figure 5. Rac activity in chemotaxing neutrophils.
During neutrophil chemotaxis, active Rac is mostly found at the leading edge of the migrating cell. This movie shows Rac activity in a neutrophil from the Rac-FRET reporter mouse (Johnsson A-K et al, 2014, Cell Rep 6:1153-64). The movie was made by FLIM-FRET time-lapse imaging of a Rac-FRET neutrophil as it migrates towards the chemoattractant fMLP on the left. Rac activity is pseudo-coloured; red = high, blue = low. (Movie from Anna-Karin Johnsson).